Note: The reader should be firstly acquainted with the article Brief introduction to the Basic Structures of Matter theory and derived atomic models.
The suggested structures are theoretically derived by analysis of the properties of the gold in different states (vapor state, "monoatomic" state and metallic state) from a point of view of BSM theory.
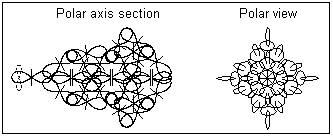
1. In a vapor state it is assumed that the golden atoms are completely separated. The polar axis section and the polar view of gold atom is shown in Fig. 1. It shows that the atomic nucleus of gold does not possesses a central symmetry. The quantum orbit connected to the single valence proton and containing a single electron is shown by a dashed line. The orbital plane orientation is perpendicular to the atomic polar axis.

Fig. 1. Gold atom in a vapor state (atomic structure)
2. The "monoatomic" state of gold is discovered by David Hudson. It is a white powder with completely different physical properties than the metallic gold. Information about David Hudson discovery and his patent is available in http://monoatomic.earth.com/david-hudson/patent-oz.html The "monoatomic" states of gold and some other transition elements is referred by David Hudson as ORME (Orbitally Rearanged Monoatomic Elemental forms). For different transition elements there is a critical number of atoms in this form. The critical number for gold is two. The BSM analysis unveils the possible configuration of "monoatomic" gold as a structure of two gold atom connected by single quantum orbits containing two electrons with oposite quantum mechanical spins. (The quantum mechanical spin of the electron according to BSM is defined by the circling direction of the electron referenced to the proton twisting). The latter is a stable atribute of the proton). Fig 2. shows the possible configuration of the "monoatomic" gold.
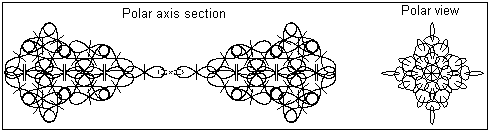
Fig. 2. A possible configuration of "monoatomic" gold
The shown structure of "monoatomic" gold should be quite stable against chemical attacks because:
the structure is symmetrical
the electronic bond is stable because it contains paired electrons and it is additionally enforced by the Intrinsic Gravitation forces (appearing as a type of Van der Waals forces).
The stable electronic bond could not supply a free electron, so this type of structure should not be conductive.
3. In metalic state the elementary atomic structure should assure the known physical properties of the gold metal: a color, molding properties, a temperature and an electrical conductivity. The BSM analysis unveils a possible existence of stable symmetrical structure comprised of six gold atoms. A plane section projection of such structure is shown in Fig. 3.
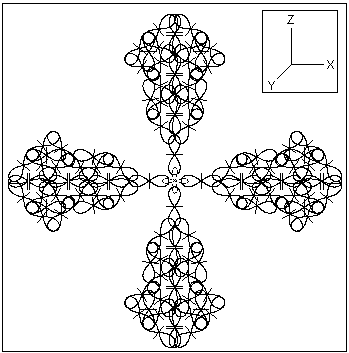
Fig. 3. A cluster of six gold atoms that could be the possible elementary structure for the metallic state of gold. The plane section corresponds to XOZ plane. The other sections in XOY and YOZ planes are identical.
One important feature of the presented cluster of six gold atoms is that it may contain only one quantum orbit in the middle of the structure but with a complex shape. In such case this complex orbit could not contain all six electrons but only two (matching the Pauli exclusion principle). Then the other four electrons will be free from the quantum orbits constrains. They may circulate not only between the different atoms in the cluster but also between the separate clusters. In such case a larger domains of clusters will still appear as neutral, while the electron gas may exhibit some constant quantum mechanical fluctuations (without applied external potential). The cluster configuration allows easy interconnections between neighboring clusters due to Intrinsic Gravitational forces (a type of Van der Waals forces). In a solid state of the matter this type of interconnection is not critical like the interconnections by quantum orbits (valid exclusively for non metallic solids) and allows a larger freedom. This feature may explain the good molding properties of the gold metal in solid aggregate state in comparison to all other metals.
4. Thin layers. In case of thin layers obtained by special epitaxial processes such as a molecular beam epitaxy, for instance, cluster structures different than the above mentioned structures could be obtained.